Absorption of water and solutes(1)
(click to download the printable version of the post, courtesy of Tiziana Marella)
Water and electrolytes
The small intestine must absorb massive quantities of water. A normal person or animal of similar size takes in roughly 1 to 2 liters of dietary fluid every day. On top of that, another 6 to 7 liters of fluid is received by the small intestine daily as secretions from salivary glands, stomach, pancreas, liver and the small intestine itself.
By the time the ingesta enters the large intestine, approximately 80% of this fluid has been absorbed. Net movement of water across cell membranes always occurs by osmosis, and the fundamental concept needed to understand absorption in the small gut is that there is a tight coupling between water and solute absorption. Another way of saying this is that absorption of water is absolutely dependent on absorption of solutes, particularly sodium:
- Sodium is absorbed into the cell by several mechanisms, but chief among them is by cotransport with glucose and amino acids – this means that efficient sodium absorption is dependent on absorption of these organic solutes.
- Absorbed sodium is rapidly exported from the cell via sodium pumps – when a lot of sodium is entering the cell, a lot of sodium is pumped out of the cell, which establishes a high osmolarity in the small intercellular spaces between adjacent enterocytes.
- Water diffuses in response to the osmotic gradient established by sodium – in this case into the intercellular space. It seems that the bulk of the water absorption is transcellular, but some also diffuses through the tight junctions.
- Water, as well as sodium, then diffuses into capillary blood within the villus.
As sodium is rapidly pumped out of the cell, it achieves very high concentration in the narrow space between enterocytes. A potent osmotic gradient is thus formed across apical cell membranes and their connecting junctional complexes that osmotically drives movement of water across the epithelium.
Water is thus absorbed into the intercellular space by diffusion down an osmotic gradient. However, looking at the process as a whole, transport of water from lumen to blood is often against an osmotic gradient – this is important because it means that the intestine can absorb water into blood even when the osmolarity in the lumen is higher than osmolarity of blood.
Monosaccarides
Simple sugars are far and away the predominant carbohydrate absorbed in the digestive tract, and in many animals the most important source of energy. Monosaccharides, however, are only rarely found in normal diets. Rather, they are derived by enzymatic digestion of more complex carbohydrates within the digestive tube.
Particularly important dietary carbohydrates include starch and disaccharides such as lactose and sucrose. None of these molecules can be absorbed for the simple reason that they cannot cross cell membranes unaided and, unlike the situation for monosaccharides, there are no transporters to carry them across. These complex sugars are indeed digested by enzymes that liberate monosaccharides, such as glucose.
Glucose generated by digestion of starch or lactose is absorbed in the small intestine only by cotransport with sodium, a fact that has exceptionally important implications in medicine.
Absorption of glucose entails transport from the intestinal lumen, across the epithelium and into blood.
Once inside the enterocyte, glucose and sodium must be exported from the cell into blood. We’ve seen previously how sodium is rapidly shuttled out in exchange for potassium by the battery of sodium pumps on the basolateral membrane, and how that process maintains the electrochemical gradient across the epithelium. The energy stored in this gradient is actually what is driving glucose entry through the sodium-dependent hexose transporter . Recall also how the massive transport of sodium out of the cell establishes the osmotic gradient responsible for absorption of water.
Glucose, galactose and fructose are tranported out of the enterocyte through another hexose transporter in the basolateral membrane. These monosaccharides then diffuse “down” a concentration gradient into capillary blood within the villus.
Body fluids and fluids’ compartments(2)
Water content of the body
Human beings are mostly water, ranging from about 75 percent of body mass in infants to about 50–60 percent in adult men and women, to as low as 45 percent in old age. The percent of body water changes with development, because the proportions of the body given over to each organ and to muscles, fat, bone, and other tissues change from infancy to adulthood (Figure 1). Your brain and kidneys have the highest proportions of water, which composes 80–85 percent of their masses. In contrast, teeth have the lowest proportion of water, at 8–10 percent.
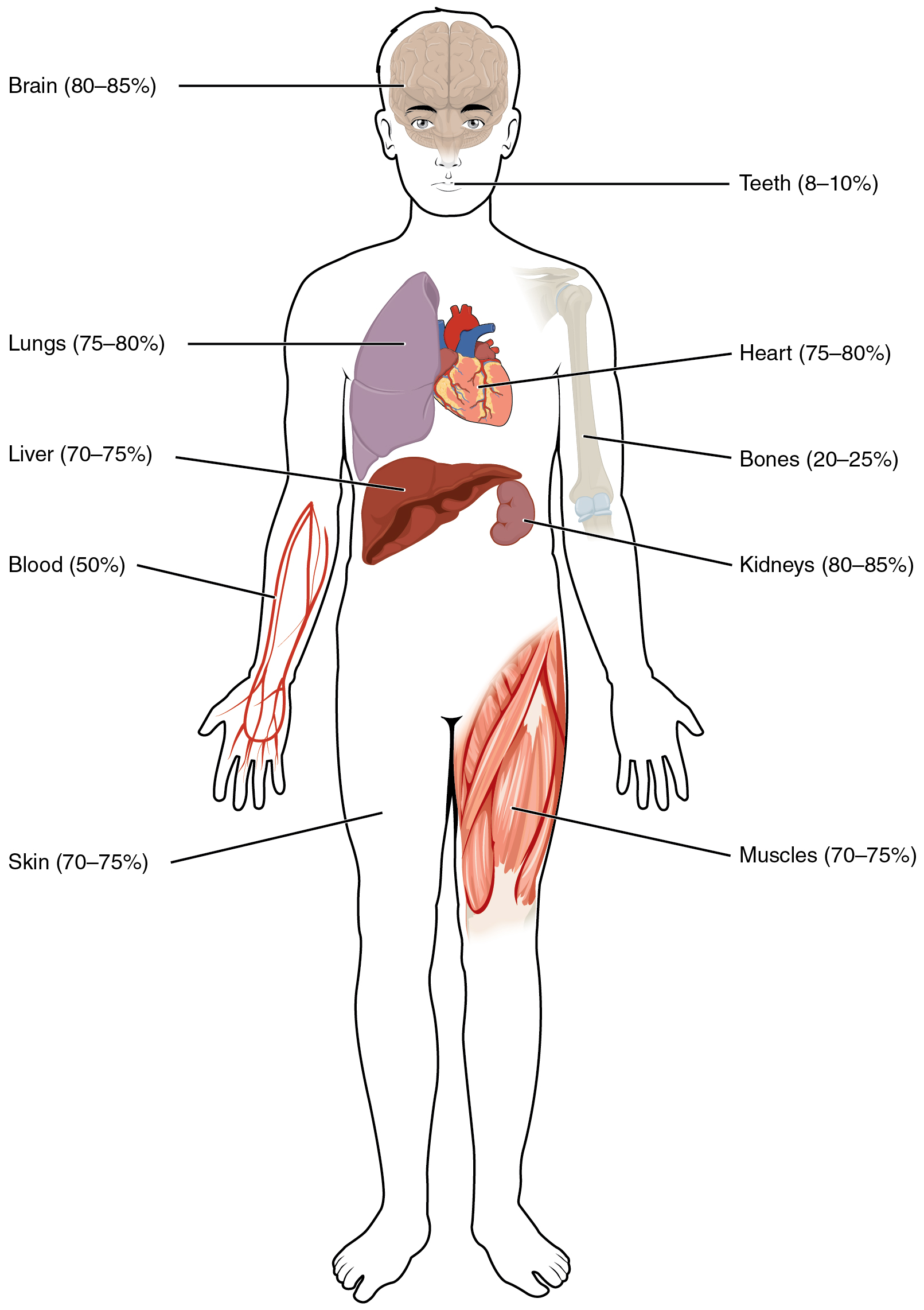
Figure 1. Water Content of the Body’s Organs and Tissues. Water content varies in different body organs and tissues, from as little as 8 percent in the teeth to as much as 85 percent in the brain.
Fluid Compartments
Body fluids can be discussed in terms of their specific fluid compartment, a location that is largely separate from another compartment by some form of a physical barrier. The intracellular fluid (ICF) compartment is the system that includes all fluid enclosed in cells by their plasma membranes. Extracellular fluid (ECF) surrounds all cells in the body. Extracellular fluid has two primary constituents: the fluid component of the blood (called plasma) and the interstitial fluid (IF) that surrounds all cells not in the blood (Figure 2).
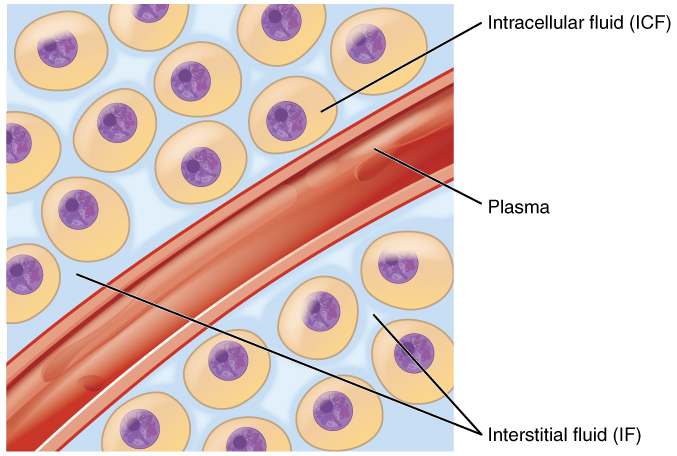
Figure 2. Fluid Compartments in the Human Body. The intracellular fluid (ICF) is the fluid within cells. The interstitial fluid (IF) is part of the extracellular fluid (ECF) between the cells. Blood plasma is the second part of the ECF. Materials travel between cells and the plasma in capillaries through the IF.
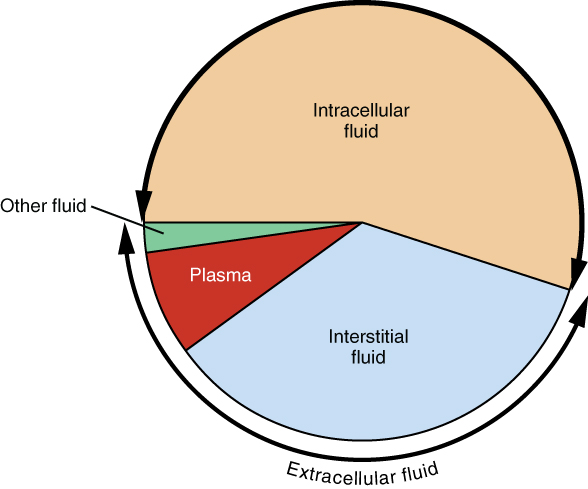
Figure 3. A Pie Graph Showing the Proportion of Total Body Fluid in Each of the Body’s Fluid Compartments. Most of the water in the body is intracellular fluid. The second largest volume is the interstitial fluid, which surrounds cells that are not blood cells.
The body has other extracellular fluid compartments. These include the cerebrospinal fluid that bathes the brain and spinal cord, lymph, the synovial fluid in joints, the pleural fluid in the pleural cavities, the pericardial fluid in the cardiac sac, the peritoneal fluid in the peritoneal cavity, and the aqueous humor of the eye. Because these fluids are outside of cells, these fluids are also considered components of the ECF compartment.
Composition of Body Fluids
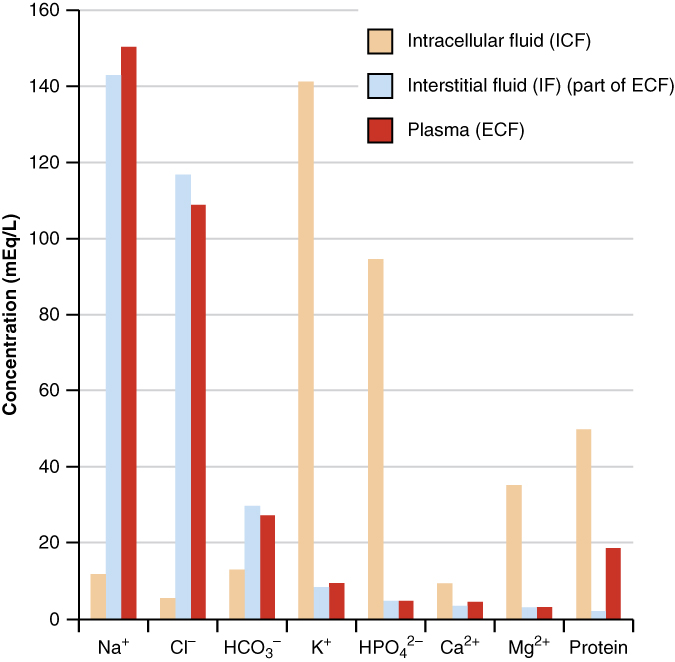
Figure 4. The Concentrations of Different Elements in Key Bodily Fluids. The graph shows the composition of the ICF, IF, and plasma. The compositions of plasma and IF are similar to one another but are quite different from the composition of the ICF.
Fluid Movement and hydrostatic pressure
Hydrostatic pressure, the force exerted by a fluid against a wall, causes movement of fluid between compartments. The hydrostatic pressure of blood is the pressure exerted by blood against the walls of the blood vessels by the pumping action of the heart. In capillaries, hydrostatic pressure (also known as capillary blood pressure) is higher than the opposing “colloid osmotic pressure” in blood—a “constant” pressure primarily produced by circulating albumin—at the arteriolar end of the capillary (Figure 6). This pressure forces plasma and nutrients out of the capillaries and into surrounding tissues. Fluid and the cellular wastes in the tissues enter the capillaries at the venule end, where the hydrostatic pressure is less than the osmotic pressure in the vessel. Filtration pressure squeezes fluid from the plasma in the blood to the IF surrounding the tissue cells. The surplus fluid in the interstitial space that is not returned directly back to the capillaries is drained from tissues by the lymphatic system, and then re-enters the vascular system at the subclavian veins.
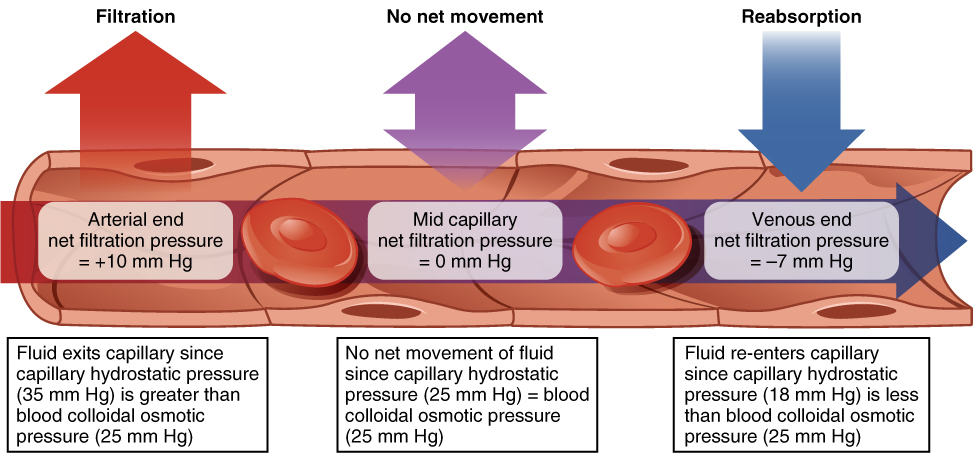
Figure 5. Capillary Exchange. Net filtration occurs near the arterial end of the capillary since capillary hydrostatic pressure (CHP) is greater than blood colloidal osmotic pressure (BCOP). There is no net movement of fluid near the midpoint of the capillary since CHP = BCOP. Net reabsorption occurs near the venous end of the capillary since BCOP is greater than CHP.
Hydrostatic pressure is especially important in governing the movement of water in the nephrons of the kidneys to ensure proper filtering of the blood to form urine. As hydrostatic pressure in the kidneys increases, the amount of water leaving the capillaries also increases, and more urine filtrate is formed. If hydrostatic pressure in the kidneys drops too low, as can happen in dehydration, the functions of the kidneys will be impaired, and less nitrogenous wastes will be removed from the bloodstream. Extreme dehydration can result in kidney failure.
Body Fluid Regulation(3)
Osmotic Pressure of Body Fluid
The osmotic pressure of body fluid is most affected by the concentration of Na+ ions present, since Na+ ions account for 90 percent of all the cations (positive ions) in the body. Note that the key word here is “concentration.” The Na+ concentration can be regulated by the amount of water retained or excreted. Three mechanisms regulate the amount of dilution.
- One is the use of antidiuretic hormone (ADH). ADH is released by the posterior pituitary gland when osmoreceptors sense a high osmolarity in the blood and tissue fluids. It acts by increasing the permeability of the distal tubules and the collecting ducts to water so that more water is reabsorbed into the blood. The effect of this is that a smaller volume of concentrated urine is produced. A person who has the disease diabetes insipidus produces no ADH and may excrete 20 liters of urine daily.
- Another mechanism that is extremely important is the countercurrent mechanism. The Medullary Osmotic Gradient allows the osmotic concentration of the blood plasma and other body fluids to be maintained around 300 milliosmol (mOsm). The juxtamedullary nephrons whose loops of Henle extend deep into the kidney medulla, are involved in this mechanism.
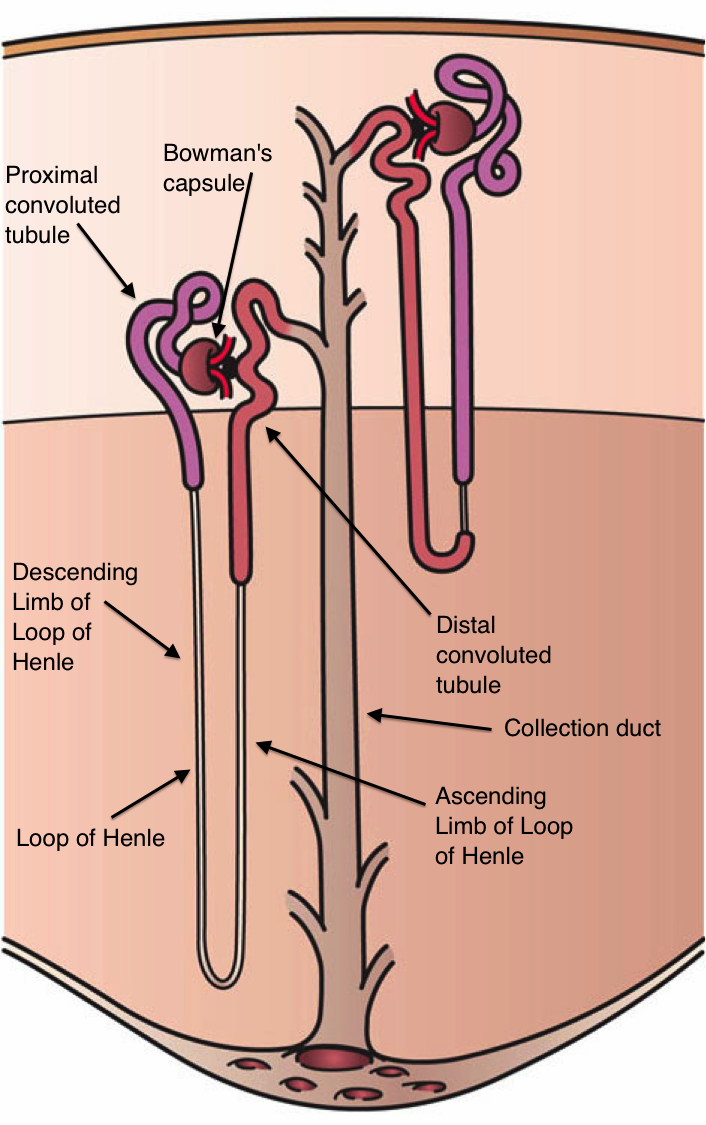
Figure 6 Juxtamedullary Nephrons
The kidney filtrate passes into the descending limb of the loop of Henle and then moves into the ascending limb of the loop. The ascending limb is impermeable to water and also responsible for actively transporting NaCl out of the nephron setting up a hypertonic conditions in the interstitial fluid surrounding the loop of Henle and the collecting duct. (Urea also moves into the interstitial fluid of the medulla and contributes to the hypertonic concentration as well.) Since the descending portion of the loop of Henle is not impermeable to water, water can move out of the descending limb of the loop of Henle in response to the osmotic gradient established by the presence of NaCl and urea in the interstitial fluid. This gradient is regulated in the kidney medulla, thus allowing to regulate the volume and osmotic pressure of the filtrate too.
- The third mechanism for controlling osmotic concentration is the thirst response. Neurons in the thirst center, located in the hypothalamus, are stimulated when the osmotic concentration of the body fluids is too high. This causes a person to feel thirsty and to drink; this increases the amount of water in the body, thus decreasing the osmotic concentration.
Regulation of pH in the Body Fluids
Acidity of body fluids is of crucial importance. The level of pH in the blood must be maintained between 7.35 and 7.45. Interstitial fluid averages a pH of about 7.35, and a pH of about 7.0 is found inside most cells. (Of course, there are parts of the body that are exceptions: the gastric glands work to produce an extremely acid environment in the stomach, the contents of the small intestine are somewhat basic, and the vaginal secretions are acidic.) Acid is produced constantly in the cells as a byproduct of metabolism. If allowed to remain, the cells would cease to function in short order.
Three basic systems mediate pH homeostasis:
1. the circulatory system
The circulatory system monitors pH by the presence of buffers in the blood. A buffer is a compound that, when added to a solution, prevents drastic changes in pH. It usually consists of a weak acid and the salt of that acid, or a weak base and the salt of that base. An example is the buffer pair H2CO3 (carbonic acid, a weak acid) and NaHCO3 (sodium bicarbonate, the salt of carbonic acid). This buffer pair is capable of neutralizing either a strong acid or a strong base:
(Strong Base)NaOH + (Weak Acid)H2CO3  (Weak Base)NaHCO3 + H2O
(Weak Base)NaHCO3 + H2O
(Strong Acid)HCl + (Weak Base)NaHCO3  NaCl + (Weak Acid)H2CO3
NaCl + (Weak Acid)H2CO3
In both instances, the strong acid or base was converted into a more neutral chemical.
2. the respiratory system
The respiratory system helps balance pH by causing a faster rate of breathing when a low pH is detected by the respiratory center in the medulla. This allows excess CO2 to be exhaled. Since CO2 lowers the pH by combining with water to form carbonic acid, eliminating CO2 helps to increase the pH.
3. the excretory (kidney) system
The excretory system assists with pH regulation by excreting H+ and reabsorbing Na+ when the pH is too low. The opposite occurs when the pH is elevated.
Fluid Volume
Volume of body fluids is regulated partially by the fact that increased fluid volume causes increased fluid pressure, which will cause more plasma to enter the Bowman’s capsules and be filtered. The renin-angiotensin system also plays an important role. A decrease in blood volume or blood pressure stimulates the kidney to secrete the hormone, renin. This hormone stimulates a eries of actions that finally lead to:
- increases Na+ reabsorption in the kidneys
- release of the antidiuretic hormone by the posterior pituitary gland
- stimulation of the thirst center in the hypothalamus.
- release of angiotensin II, which is a strong vasoconstrictor affecting the smooth muscle of the arterioles.
Regulation of Specific Ions
An example of regulation of specific ion concentration is given by the Calcium (Ca2+) and Phosphate (PO42-) balance.
When the calcium concentration in the blood and tissues drops, parathyroid hormone is secreted and produces the following effects:
-
-
- it causes increased release of Ca++ and HPO4 2- from the bone because it stimulates osteoclast cells to digest the bone;
- in the small intestine, it causes increased reabsorption of Ca++ (Intestinal reabsorption of Ca++occurs because parathyroid hormone stimulates the kidneys to activate vitamin D, which is necessary for Ca++ reabsorption in the intestine;
- in the nephrons, it induces increased reabsorption of Ca++ while it inhibits reabsorption of PO4 Thus calcium (Ca++) ions are reabsorbed while phosphate (HPO4 2-) ions are excreted by the kidneys.
-
Figure 5 Effect of parathyroid hormone on bone, the kidneys and the intestine
When calcium level in the blood rices the parathyroid hormone is no longer secreted.
The hormone calcitonin has a completely different effect on calcium balance, working to remove Ca++ from the blood and deposit it in the bone. Secreted from the thyroid gland especially during children growth, pregnancy and lactation , calcitonin stimulates the bone-building osteoblasts which deposit Ca++ in bone while it also inhibits the bone-digesting osteoclasts.
How to keep a healthy fluid balance
References
-
-
- Hypertext for biomedical Sciences – Colorado State University (text was copied from the following paragraphs with minor modifications and omissions: absorption of water and electrolytes; absorption of monosaccharides)
- BCcampus Open Textbook Project (text was adapted from https://opentextbc.ca/anatomyandphysiology/chapter/26-1-body-fluids-and-fluid-compartments/)
- Penn State Eberly College of Science – Introduction to physiology (text was adapted from https://online.science.psu.edu/biol141_wc/node/7612)
-






Pingback: Fluid Spacing and Regulation of Water Balance - Overall Science
Pingback: My Homepage